How Many Atoms Constitute One Unit Cell of a Face-centered Cubic Crystal. A simple cubic unit cell is also known as primitive cubic unit cell.
Unit Cell Simple Cubic Body Centered Cubic Face Centered Cubic Cryst Unit Cell Crystal Lattice Structure Nomenclature Chemistry
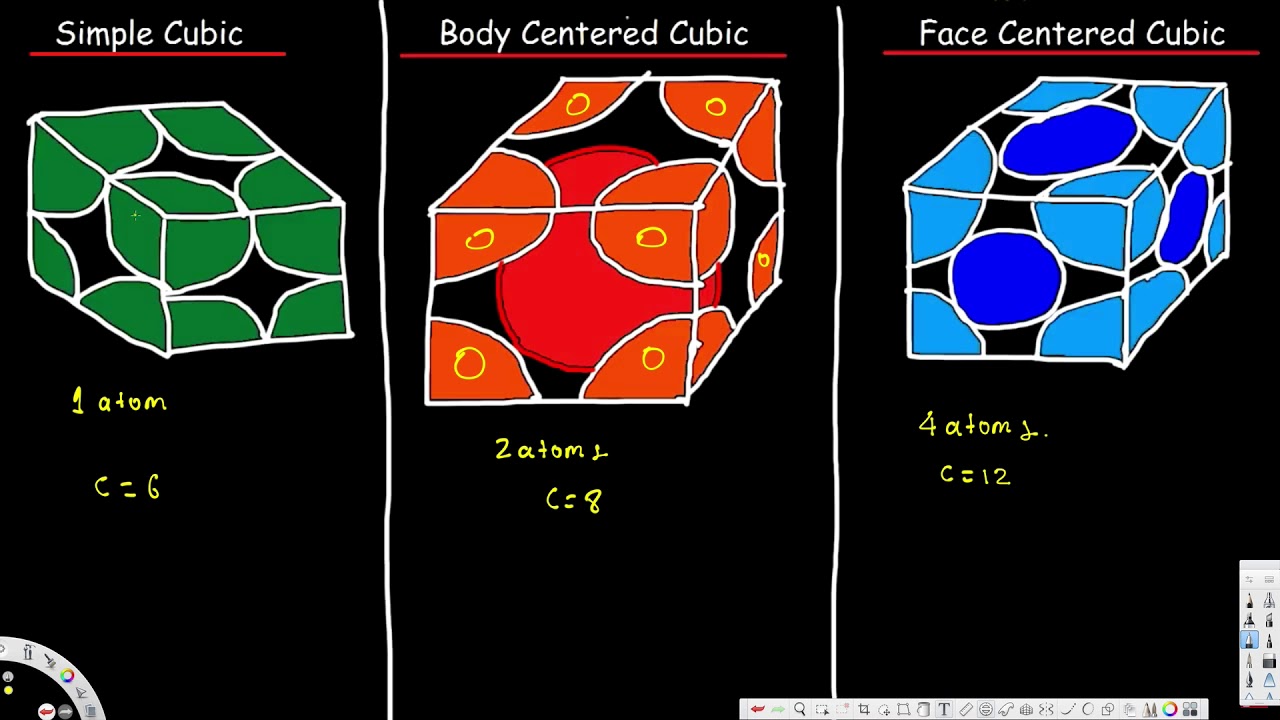
The total number of atoms in a simple cubic primitive cubic unit cell is 8181 atom.

. How Many Atoms In A Simple Cubic Unit Celleight atomsHow many atoms are in a cubic unit celleight atomsThe smallest repeating unit of a crystal lattice is the unit cell. 2 2 atoms are in body-centered cubic unit cell. Each atom contributes one eigth to the unit cell.
Simple cubic unit cell has atoms at its corners each of which is shared between eight adjacent unit cells. How many atoms of Au are present in each unit cell. View the full answer.
How many atoms contained in face centered cubic unit cell. How many atoms contained in body centered cubic unit cell. B In terms of the lattice constant a what is the distance between nearest-neighbor atoms in a simple cubic lattice.
8 atoms are present at 8 corners of a simple cubic unit cell. Write the names and chemical formulae of any one ore of zinc. In the unit cell characterizing the diamond lattice.
In a fcc unit cell. A unit cell is the smallest repeating portion of a crystal lattice. It has 8 atoms at each of its corners.
1 simple cubic 2 face-centered cubic and 3 body-centered cubic. Electrical Engineering questions and answers. 8 atoms are present at 8 corners of a fcc unit cell.
These are corner atoms so each one only contributes one eighth of an atom to the unit cell thus giving us only one net atom. Total contribution 8 8 1 1 2 The number of atoms contained in one face-centred cubic unit cell of monoatomic substance is 4. 5 rows The net sum of atoms in a Face-Centred Unit Cell 1 3 4 atoms.
Each atom contributes one eigth to the unit cell. In a bcc unit cell. Therefore the total number of atoms in a unit cell 4 atoms.
The total number of atoms present in a simple cubic unit cell is one. What is repeating order of layers in each. How many atoms of Mo are present in each unit cell.
The simple cubic unit cell is delineated by eight atoms which mark the actual cube. A How many atoms are there in a simple cubic unit cell. We review their content and use your feedback to keep the quality high.
These are shown in three different ways in the Figure below. 100 22 ratings 1 1 atom in simple cubic unit cell. 8 corners x 18 1 atom.
3 4 atoms are in face-ce. A unit cell is the smallest representation of an entire crystal. Volume of HCP Unit Cell.
The hexagonal closest packed HCP has a coordination number of 12 and contains 6 atoms per unit cell. If nickel formed a body-centered cubic structure there would be two atoms per unit cell because the nickel atom in the center of the body wouldnt be shared with any other unit cells. The face-centered cubic FCC has a coordination number of 12 and contains 4 atoms per unit cell.
Molybdenum has a body-centered cubic unit cell. As one example the cubic crystal system is composed of three different types of unit cells. A six sided cube with a sphere on each corner and a line along each edge of the cube connecting the center of each sphere with the centers of three other spheres.
This unit cell uses nine atoms eight of which are corner atoms forming the cube and one more in the center of the cube. Unit cells occur in many different varieties. Experts are tested by Chegg as specialists in their subject area.
What is repeating order of layers in each packing of simple cubic unit cell. The total number of atoms present in a simple cubic unit cell is one. The corners contribute only one net atom and.
Gold has a face-centered cubic unit cell.

Unit Cell Face Centered Cubic Crystal Lattice Structures Crystal Lattice Structure Unit Cell Nomenclature Chemistry

Unit Cell Chemistry Atomic Radius Density Edge Length Calculations Unit Cell Nomenclature Chemistry Chemistry
0 Comments